Company
Gintzler International is a leading narrow web label, shrink sleeve, and product identification printer and converter, specializing in servicing the prime consumer product and pharmaceutical packaging industries. Clients include Mabamex (Mattel Toys), Sorrento Cheese, Green Mountain Coffee Roasters, as well as several pharmaceutical customers. Gintzler’s state-of-the-art production facility operates under cGMPs.
Situation
Gintzler International was not certified to a formal quality management system, but desired to compete in markets that required certification to the ISO 13485 medical device standard. Management felt ISO 13485 certification would provide them significant improvement and growth opportunities.
Solution
Insyte Consulting performed a gap analysis between the company’s current quality system and the requirements of ISO 13485 to determine Gintzler’s readiness to implement the standard. After reviewing the results from the analysis, the company committed to pursuing ISO 13485 certification.
An Insyte-led project team utilized the gap analysis to make the required upgrades to the quality system. All personnel were trained on the requirements and expectations of ISO 13485, and procedures were written and adopted by the workforce. A team of internal auditors was trained, and audits of the new system were initiated. This proved particularly effective in spreading the use and knowledge of the quality management system. In addition, management review meetings drove continual improvement of the system, product quality and company performance.
Simultaneously, Gintzler decided to implement a new Enterprise Resource Planning (ERP) system. Management believed this new ERP system would better manage quality system documentation and records, as well as enhance data analysis and information sharing. Once the new ERP system was in place and the quality management system was upgraded accordingly for consistency, Insyte performed a mock audit to ensure compliance to the ISO 13485 standard.
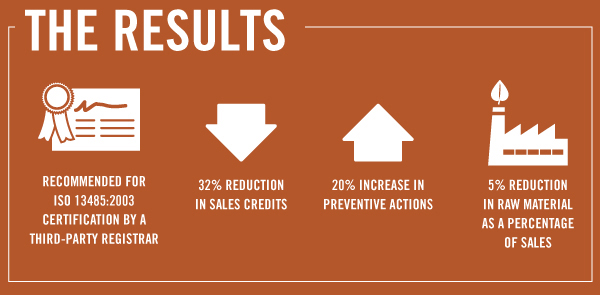
Gintzler’s certification audit was successful, with minor nonconformances that were quickly addressed; the company was recommended for certification.